What is immunotherapy?
Immunotherapy is a treatment aimed at targeting and overcoming cancer through the immune system of the body.
There are two main types of immunity. “Adaptive immunity” which requires exposure to a specific target or antigen in order to attack (such as in vaccination strategies), and “innate immunity” which is a nonspecific mechanism to fight off any foreign, infected or abnormal cell.
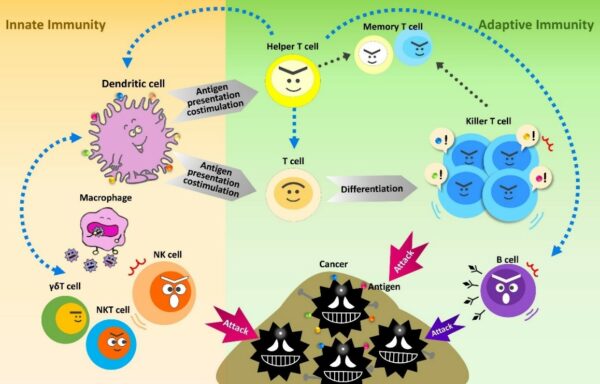
The immunotherapy we offer is a multivalent Dendritic Cell Vaccine that specifically attacks cancers through adaptive immunity. We combine this treatment with an activated Natural Killer cell therapy (or activated NK / NKT /γδT cell therapy) which uses the innate immune pathway. By targeting cancer cells through both pathways, maximal specific recognition of the cancer cells is ensured, without the destruction of healthy normal cells.
Tumors are not just a clone of a single cell type, but contain a diverse population of cancer cells. In order to attack the various types of cancer cells without tumor escape (evading the immune system), a treatment that uses both adaptive immunity and innate immunity to its advantage, is most ideal. Our hybrid therapy, combining our multivalent Dendritic Cell vaccine with the activated NK cell therapy has been considered a “secret weapon” in cancer vaccination strategies.
As the treatment uses the patient’s own immune system to specifically attack the cancer at the cellular level, there are minimal to no side effects. Treatment can be safely obtained while maintaining and improving the quality of life (QOL).
Features of Targeted Cellular Immunotherapy
- There are minimal to no side effects, as treatment uses the patient’s own immune system.
- There is no need for hospitalization, treatment is done as an outpatient in the clinic.
- This treatment maintains and/or improves the quality of life for the patient.
- This treatment can be used in combination with surgery, radiation, or chemotherapy.
- Can be effective even for invasive cancers, progressive and terminally ill patients.
- Can be effective to prevent recurrence and metastasis.
- Life prognosis can be expected to improve.
- Unfortunately, having poor nutritional status reflected by low albumin levels, not having basic physical strength, such as inability to walk or being bedridden indicates a weakened immune system and the effects of the treatment may not be as expected. However, even in such a weakened state, benefits such as increased appetite, energy, vitality, and pain reduction may significantly improve QOL.
Patented Technology
In our pursuit for a better cancer treatment – a safer, more effective treatment, free of side-effects – we arrived at a solution using the body’s own immune system. Since the year 2000, we have been steadily researching and developing technologies and have achieved excellent treatment results, attaining patents in various countries around the world.
In particular, in a series of groundbreaking technology patents related to the production of dendritic cell vaccines, we have been able to make strides to significantly reduce the burden on the patient’s body during the treatment. The multivalent dendritic cell vaccine we provide continues to be upgraded using patented platform technology.
- Apheresis (extra-corporeal whole blood component collection) is unnecessary.
- Using undifferentiated monocyte growth technology, each vaccine is produced using only 25 ml of venous blood.
- We only use cancer antigens that are long overlapping peptides.
・These antigens are HLA unrestricted as they are taken up into dendritic cells by endocytosis
・Our dendritic cell vaccine presents antigens to MHC class I and class II receptors, which activates both CD4+ helper and CD8+ killer T cells.
・CD8+ killer T cells differentiate into aggressive effector T cells. - A minimum of four to eight tumor antigens (peptides) are incorporated into the vaccine.
・This provides multiple targets to counteract the diversity of cancer cells.
・If cancer tissue is available, we can create a tumor specific antigen with tumor lysate. - Heat shock protein (HSP70) is used in the creation of the vaccine.
・The HSP70 receptor (CD91) works as a chaperone to efficiently incorporate antigens. - NK cells are extracted from the same blood collection for the dendritic cell vaccines, and are proliferated and activated in large quantities.
・Hybrid therapy, using NK cells in conjunction with the dendritic cell vaccine, works synergistically to prevent immune escape by using both the innate and adaptive immune system.
Patents held
Culture method for the activity and proliferation of lymphocytes
Japanese Patent No. 4275680
Monocyte proliferation, monocyte growth medium, monocyte growth production method, dendritic cell production, dendritic cell vaccine production method
Japanese Patent No. 5577472
U.S. Patent US 9303247 B2
European Patent EP 2749639 B1 *Germany, United Kingdom, France
Korea Patent No. 10-1680164
Singapore Patent SG11201404570W
Taiwan Patent I607017
China Patent CNZL201380001273.1
Macao Patent MOJ/003854
Method to obtain monocytes and/or NK cells
Japanese Patent No. 5856025
Korea Patent No. 10-1732532
U.S. Patent US 10113148 B2
Method for undifferentiated proliferation of mesenchymal stem cells, and method of enriching mesenchymal stem cells
Japanese Patent No. 5856029
Canadian Patent No. 2872852
U.S. Patent US 9670461 B2
Korea Patent No. 10-1813269
European Patent EP 2891713 *Germany, United Kingdom, Italy
Method for producing composition of immune cells and composition of cells for cancer treatment
Japanese Patent No. 5997296
Taiwan Patent No. I612137
Korea Patent No. 10-1923848
U.S. Patent US 10160952 B2
Book Release
 Ovarian Cancer Immunotherapy, Edited by Samir A. Farghaly, Oxford University Press, 2018
Ovarian Cancer Immunotherapy, Edited by Samir A. Farghaly, Oxford University Press, 2018
Chapter 9: Heat Shock Protein Vaccine Therapy for Ovarian Cancer
Hiroyuki Abe, Amane Sasada, Shigeki Tabata, and Minako Abe
“Ovarian Cancer Immunotherapy” was published on November 22, 2018 by the Oxford University Press, UK. Each chapter of this book is written by a team of prominent medical institutions from around the world and is written from an overview of basic science and clinical treatments of immunotherapy in ovarian cancer.
Our medical team was asked to contribute and write one of the chapters, entitled “Heat Shock Protein Vaccine Therapy for Ovarian Cancer”. As ovarian cancer often arises initially without overt symptoms, only about thirty percent are found in the early stages. In approximately seventy percent of cases, the ovarian cancer has already progressed to advanced stages by the time of diagnosis. While there has been some progress in the use of chemotherapy, advanced ovarian cancer remains difficult to treat, and the five-year mortality rate remains high. As new treatments are being explored, the use of immune cells and immunotherapy is considered to be among the rising stars of a promising new treatment. In our chapter, we are discuss our newly developed multivalent dendritic cell vaccine which incorporates heat shock proteins as a part of the therapeutic effect.
Three Benefits of Immunotherapy at the Tokyo Cancer Clinic
1. Treatment is possible, no matter what stage or what type of cancer
As each cellular treatment is created specifically for each individual, according to the characteristics of each patient’s cancer, it is possible to receive treatment regardless of the type or stage of cancer.
Efficacy of treatment
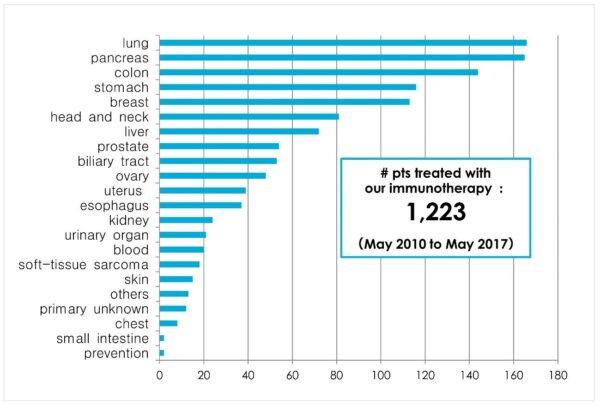
2. The double effect of both cancer reduction and increased long-term survival
Patients who have received our immunotherapy have been confirmed to have a reduction of their cancer and increased long-term survival. For example, when our multivalent dendritic cell cancer and activated NK cells were given to a patient with right sided lung cancer (metastasized from the left lung after radiation therapy), the cancer completely disappeared and tumor markers also normalized. Even in cases where standard treatments have failed, prolonged life expectancy and long term survival have been shown in colon cancer, lung cancer, pancreatic cancer, prostate cancer, breast cancer, and ovarian cancer.
Case: 89 year old female with metastatic right lung cancer after radiation of left lung cancer
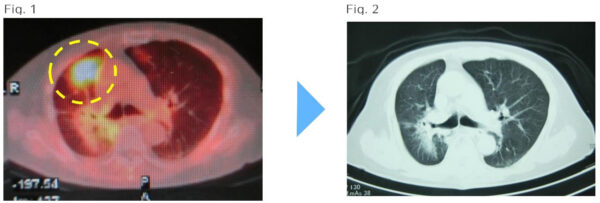
(Fig.1)May 19, 2014: (Before treatment) The encircled area shows cancer metastasis of the right lung.
(Fig.2)August 20, 2014:(After 1 round (kur) of treatment) Complete disappearance of the cancer
- Treatment flow
Approximately 25ml of venous blood is collected. The cellular therapies are individually created over 14 days, and the multivalent dendritic cell vaccine is administered by intradermal injection, and the activated NK cells are intravenously infused over 30 minutes. This process is repeated five times to complete 1 round (kur)of treatment. - Side Effects
In rare cases, a strong immune response may cause a fever of 37-38˚C within hours of administration, or the injection site may become red and swollen. In this case there were no side effects.
Long-term survival and clinical efficacy in patients with advanced lung cancer (22 patients) who have failed standard treatment.
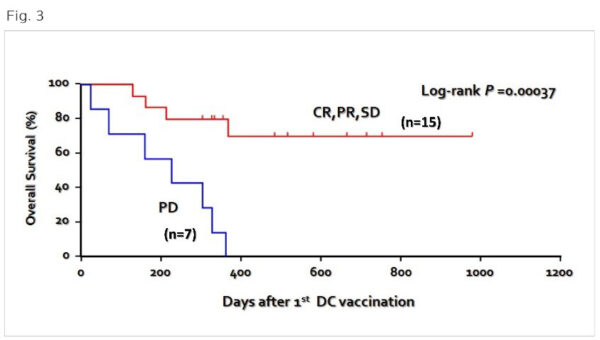 (Fig. 3) When our multivalent dendritic cell vaccine was administered to 22 patients with advanced lung cancer who had failed standard treatment, about 68% responded to the vaccine with a long-term survival rate of more than 900 days. Even for those who did not respond adequately to the vaccine, the survival rate of more than 300 days was about 32%, confirming a life-prolonging effect.
(Fig. 3) When our multivalent dendritic cell vaccine was administered to 22 patients with advanced lung cancer who had failed standard treatment, about 68% responded to the vaccine with a long-term survival rate of more than 900 days. Even for those who did not respond adequately to the vaccine, the survival rate of more than 300 days was about 32%, confirming a life-prolonging effect.
3. Our immunotherapy can be used in combination with surgery, chemotherapy and radiation therapy.
Depending on the patient’s wishes and condition, immunotherapy can be used before, after, or concurrently with standard treatment. Our cellular immunotherapy not only enhances the strength of the immune system, but also has the effect of improving quality of life (QOL), and as such the side effects of chemotherapy and radiation therapy may be reduced. In addition, when performed after standard treatment, it can be used as an effective preventive measure against cancer metastasis and recurrence.
Case: 74 year old female with Stage IIIC Ovarian Cancer
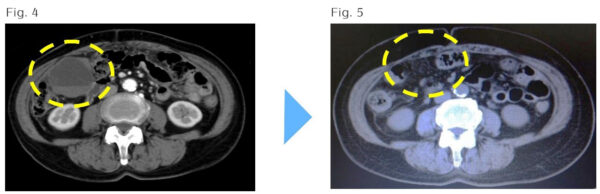
(Fig. 4) May 25, 2015: Abdominal CT after 1 round (kur) of treatment
1 round (kur) of multivalent dendritic cell vaccine and activated NK cells were administered while the patient continued chemotherapy after surgery. The mass is significantly smaller and blood test data have improved.
(Fig. 5) July 21, 2016: Abdomen CT after completion of all treatments
Three more rounds of chemotherapy were added. PET-CT showed a residual mass, but metabolic activity of cancer cells was not observed. Thereafter, the residual tumor was surgically removed, and the complete disappearance of the cancer was confirmed.
- Treatment flow
Approximately 25ml of venous blood is collected. The cellular therapies are individually created over 14 days, and the multivalent dendritic cell vaccine is administered by intradermal injection, and the activated NK cells are intravenously infused over 30 minutes. This process is repeated five times to complete 1 round (kur)of treatment. - Side Effects
In rare cases, a strong immune response may cause a fever of 37-38˚C within hours of administration, or the injection site may become red and swollen. In this case there were no side effects.
