Enhancing adaptive immunity
Patented Multivalent Dendritic Cell Vaccine
Dendritic Cells
Dendritic cells were first discussed in 1868, but the complete discovery of its capabilities and full-scale research was conducted by Dr. Ralph Steinman and others from Canada. In 1973, Steinman identified these special immune cells and their powerful ability to present antigens to lymphocytes to induce a specific immune response. He named them dendritic cells from the Greek word dendreon due to its striking “tree-like” morphology.
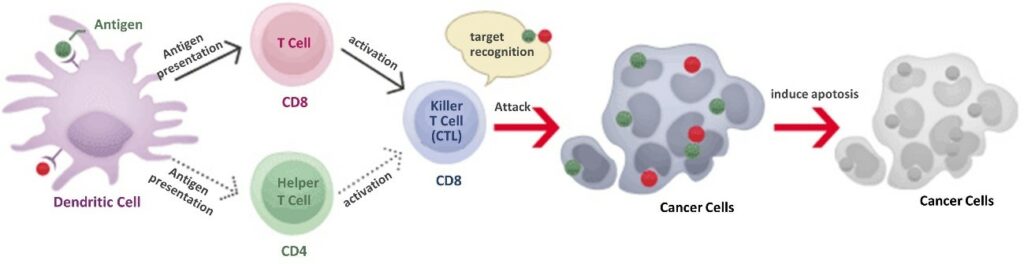
Antigen presenting ability, means the ability of immune cells to recognize viruses, bacteria and cancer cells as foreign matter. Antigen presenting cells can take in this foreign substance, disassemble it, and then present a portion of this on a special receptor on the cell surface as an antigen (a marker that it is foreign matter).
There are other immune cells have the ability to present antigens, however dendritic cells are 100 times more potent in the ability to induce an immune response. Dendritic cells are part of the innate immune system but also orchestrates the adaptive immune system. Because of this it is known as the “control tower” of the immune system, for both its powerful antigen presentation ability and its command over activating the immune system.
In 2011, Dr. Steinman was awarded the Nobel Prize in Physiology or Medicine for his outstanding contribution in his “discovery of the dendritic cell and its role in adaptive immunity”.
Multivalent Dendritic Cell Vaccine
The multivalent dendritic cell vaccine is a cellular immunotherapy that utilizes the potent antigen presentation ability of dendritic cells. Dendritic cells are responsible for providing T cells with the tumor antigens (markers of cancer), the information required to selectively target and attack cancer cells. T cells armed with this tumor antigen information can, with pinpoint accuracy, attack only the cancer cells. Normal cells are unharmed, so there are very few side effects, and because there is little burden to the body, treatment is possible even for the frail or elderly. Because cancer cells are attacked at the cellular and molecular level, invasive cancers that are difficult to remove surgically, and cancer cells that are in the lymphatic system and bloodstream can also be successfully targeted. These features make this treatment effective even for the prevention of recurrence and metastasis of cancers.
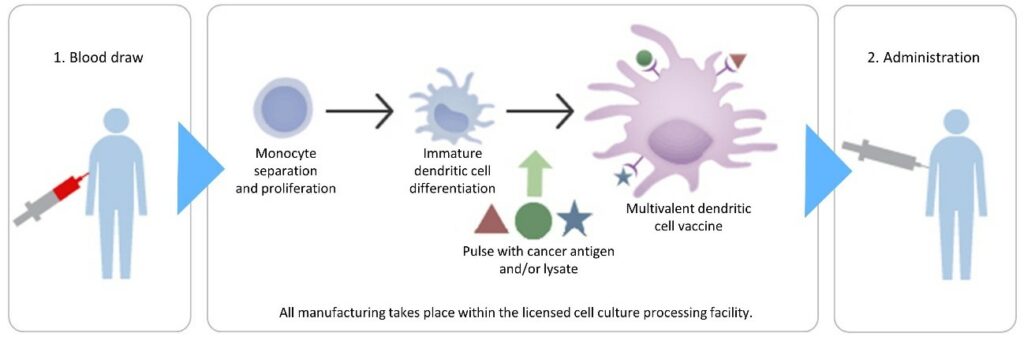
We individually create the vaccines through our patented technology using only a small amount of the patient’s own blood. This multivalent vaccine is personalized, incorporating 4 to 8 different cancer antigens in order to correspond to each patient’s specific cancer.
Treatment flow
1. Medical Consultation
Our physicians will consult with patients and in order to understand the patient’s full information impacting treatment. All factors including diagnostic imaging tests, bloodwork, medical history, current physical problems, work, lifestyle, family will be taken into consideration while recommending the best treatment and joint plan for each individual.
2. Initial testing
For the multivalent dendritic cell vaccine, initial testing including HLA testing, tumor markers, and infectious disease testing will be performed. Other testing including genetic testing, immune response testing (immunogram), CTC (circulating tumor cells) and cell free DNA testing can also be done upon request.
3. Blood draw and treatment administration
A small amount of venous blood (25ml) is collected to create the dendritic cell vaccine. The tailor-made multivalent vaccine is created in 14 days, and then can be administered. This blood drawing and administering of therapy is repeated a total of six times every 2 weeks for one round (kur) of therapy.
* When activated NK cell therapy is used in combination with the vaccine, one round (kur) of therapy is considered to be five doses.
* Due to our patented technology, apheresis (component blood collection) is not required.
* Blood collection is done by normal venipuncture.
* The vaccine is administered by intradermal injection.
4. Evaluation of treatment
At our clinic, we evaluate the patient’s progress and efficacy of treatment at baseline prior to treatment, immediately after the end of the first round, and then again 3 months after the first round of treatment. Prior to and at the end of the treatment course, we evaluate the patient’s QOL, immune response test (immunogram), tumor markers, and imaging studies. 3 months after completion, the patient’s QOL is reevaluated, the immunogram, tumor markers, and imaging studies are monitored in consultation with the patient to determine a future plan.
Side effects
As the multivalent dendritic cell vaccine is created using the patient’s own immune cells, and the cancer killing effect is done by the patient’s own immune system, there are little to no side effects. Rarely, a strong immune response may cause a fever of 37-38˚C within hours of administration, or the injection site may become red and swollen, however such reactions usually subside within 24 hours.
Targeted medical conditions and symptoms
- Cancer (any type of cancer, any stage of cancer)
- Cancer prevention, recurrence or metastasis prevention
